From smartphones to your wireless mouse, to laptops or even external batteries to charge other devices, lithium-ion batteries have been in our daily lives for some time now. In this article we are going to go into the operation of this type of batteries, because knowing how it works , you will be able to know in a better way how to use them, take care of them and prolong their useful life to the maximum.
With all the wireless devices that we have in our day to day, the autonomy of the batteries is one of the biggest headaches for designers, and so much is tried to promote their capacity and autonomy that now the problems have been further aggravated by of the systems with fast charge, because now autonomy is no longer everything, but users also want it to take as little time as possible to charge to be able to use the devices again.

So how do lithium ion batteries work? We will explain it in the simplest possible way so that you can understand its operation, as well as the risks involved.
How do lithium batteries work?
Batteries come in many shapes, colors, and sizes. As we said at the beginning we have laptop batteries, which are the largest of this internal design and there are tiny ones, such as those that your wireless gaming mouse has. But they all have in common their internal structure, made up of the following elements:
- Anode (positive electrode) : it is made of lithium cobalt oxide (or lithium phosphate, lithium magnesium …). The metal is in sheet form and adhered to the other two main components, also in the sheet.
- Cathode (negative electrode) : it is generally made of porous carbon.
- Separator : it is a sheet that separates the anode from the cathode to avoid a short circuit, and as a general rule it is made of insulating plastic.
- Electrolyte : it is an organic solvent in which the anode, cathode and separator are immersed. It is a highly flammable liquid in which lithium salts are diluted, which is why batteries are so dangerous (or, at least, they carry certain risks).
- Converters and regulators : Precisely because lithium batteries are dangerous, sensors are often integrated to measure temperature and overloads. In short, various security mechanisms are incorporated to avoid problems.

As we said, the positive electrode (anode) is made of lithium cobalt oxide as a general rule (LiCoO2), while the negative electrode (cathode) is carbon. When the battery is charged, the lithium ions move from the positive to the negative electrode through the electrolyte, binding to the carbon. During discharge, the opposite process, the lithium ions separate from the carbon to rejoin the anode. This movement of lithium ions is what generates the energy in the batteries .
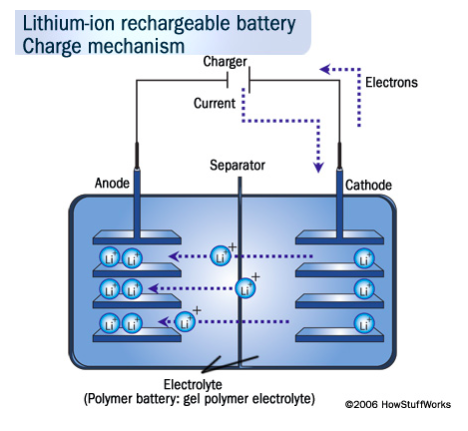
This movement of lithium ions, or electrons, produces the energy consumed by our devices but also generates heat, like any other moving component. This is why temperature is a crucial factor not only for good performance but also for safety.
Most batteries have a metal housing with a pressure sensitive vent hole. When the battery heats up, the pressure increases and to prevent it from exploding, the extra pressure is released through said hole (that’s why you will see that in many there is a warning that tells us that we should never plug that hole). Among the safety systems of lithium-ion batteries, the PTC (Positive Temperature Coeficient, or positive temperature coefficient), which is in charge of monitoring the temperature of the battery to avoid overheating, also stands out.
Why do batteries sometimes explode?
We have already told you that there are security systems to prevent this as far as possible, but sometimes these are not enough, or due to adverse environmental conditions they can fail. Let us start, however, from the premise that lithium batteries are safe since they incorporate these safety systems, but they still have within them that highly flammable material called electrolyte. If a battery explodes, it is always due to excess temperature, at which time the pressure increases, the casing gives way and burns the electrolyte.
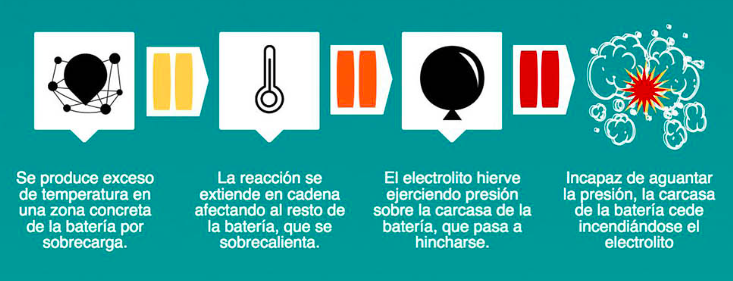
For this reason it is very important to keep batteries, whatever type and device they are, in a place that is as cool and dry as possible, since overheating can cause an explosion, and it is not a joke.